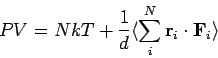In the previous exercises we have used the number of particles in the left
side of the box, and the measure of the temperature ![]() to characterize the
equilibrium of the system. Other parameter that can be used are the mean
pressure
to characterize the
equilibrium of the system. Other parameter that can be used are the mean
pressure ![]() and as well as the total energy
and as well as the total energy ![]() .
.
Other thermal quantity is the heat capacity
![]() .
. ![]() is a measure of the amount of heat needed to produce a
change in the temperature. Since this is and extensive quantity (depends on
the size of the system) it is convenient to use the specific heat
is a measure of the amount of heat needed to produce a
change in the temperature. Since this is and extensive quantity (depends on
the size of the system) it is convenient to use the specific heat
![]() instead. The easiest way to obtain it is to determine the
mean potential energies at neighboring temperatures
instead. The easiest way to obtain it is to determine the
mean potential energies at neighboring temperatures ![]() and
and ![]() .
.
In order to determine the mean pressure, suppouse for a moment that the
container has rigid walls. We know that the
collisions of particles with the walls will cause a mean net force to be
exerted on each element of the wall. The mean force per unit of area is the
pressure ![]() of the gas. The force can be found by relating the force in the
horizontal direction to the rate of change of the linear component of the
linear momentum of the particles hitting the wall.
of the gas. The force can be found by relating the force in the
horizontal direction to the rate of change of the linear component of the
linear momentum of the particles hitting the wall.
We can use a similar argument to calculate the pressure in the absence of
walls. Since the pressure is uniform at equilibrium, we can relate the
pressure to the transfer of momentum across an element of area anywhere in
the system. Consider an element of area ![]() and let
and let
![]() be the
mean momentum crossing the surface per unit of time from left to right, and
let
be the
mean momentum crossing the surface per unit of time from left to right, and
let
![]() be the mean momentum crossing the surface per unit of
time from right to left. The the mean force
be the mean momentum crossing the surface per unit of
time from right to left. The the mean force ![]() is
is
An alternative way of calculating the pressure is from the virial theorem: