


Next: Simple transport properties
Up: Evaluation of observables
Previous: Exercise 4.6: Ground state
- Choose the parameters
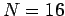 ,
, 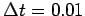 ,
, 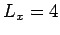 , and
, and
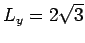 . Place the particles on a triangular lattice which fills the container
and give each particle initial zero velocity. Measure the temperature and
pressure as a function of time and plot several ``snapshots'' of the system.
What is the total energy of the system? Does it remain a solid?
. Place the particles on a triangular lattice which fills the container
and give each particle initial zero velocity. Measure the temperature and
pressure as a function of time and plot several ``snapshots'' of the system.
What is the total energy of the system? Does it remain a solid?
- Give the particles a random initial velocity with
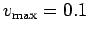 .
What is the total energy? Equilibrate the system for approximately 50 time
steps and average the pressure and temperature over 100 time steps.
.
What is the total energy? Equilibrate the system for approximately 50 time
steps and average the pressure and temperature over 100 time steps.
- Pick an equilibrium configuration from the previous item and rescale
all velocities by a factor 2. What is the new total energy? Take snapshots
of the system every 25 time steps and describe the behavior of the motion of
the particles. What is the equilibrium temperature and pressure of the
system? After equilibrium is reached, rescale the velocities again in the
same way. repeat this rescaling and measure
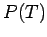 and
and 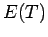 for size
different temperatures.
for size
different temperatures.
- Plot
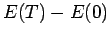 and the pressure
and the pressure 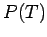 as a function of
as a function of 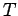 . What
is the dependence of difference
. What
is the dependence of difference 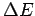 with
with 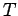 ?
?
- Decrease the density by multiplying
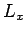 ,
, 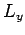 , and the particle
coordinates by a factor
, and the particle
coordinates by a factor 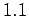 . What are your results for temperature and
pressure? What is the nature of the snapshots? Continue rescaling the
density and the positions until the system ``melts''. What is your
qualitative criterion for melting?
. What are your results for temperature and
pressure? What is the nature of the snapshots? Continue rescaling the
density and the positions until the system ``melts''. What is your
qualitative criterion for melting?



Next: Simple transport properties
Up: Evaluation of observables
Previous: Exercise 4.6: Ground state
Adrian E. Feiguin
2004-06-01