


Next: Exercise 4.6: Ground state
Up: Evaluation of observables
Previous: Evaluation of observables
In this problem we compute the pressure and hence the ``equation of state''
(the relation between pressure, temperature and volume) of a gas
- Consider
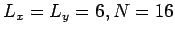 . Choose the initial configuration to be an
equilibrium configuration. Compute the mean pressure using the net momentum
transfer method and the virial theorem. Allow at least 100 time steps for
equilibration and 200 to compute averages. Is the pressure constant or
fluctuates? How does the pressure compare with the ideal gas result? Which
method of computing the pressure is more accurate?
. Choose the initial configuration to be an
equilibrium configuration. Compute the mean pressure using the net momentum
transfer method and the virial theorem. Allow at least 100 time steps for
equilibration and 200 to compute averages. Is the pressure constant or
fluctuates? How does the pressure compare with the ideal gas result? Which
method of computing the pressure is more accurate?
- An approximate equation of state for dense gases and liquids is the
``van der Waals'' equation:
The phenomenological parameters 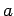 and
and 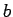 are related to the repulsive and
attractive parts of the interaction respectively and are approximately
independent of the temperature.. Use the same configuration to find the
dependence of
are related to the repulsive and
attractive parts of the interaction respectively and are approximately
independent of the temperature.. Use the same configuration to find the
dependence of 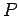 on
on 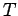 . Plot
. Plot 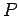 versus
versus 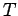 and use the van der Waals
equation to obtain an estimate for the parameters
and use the van der Waals
equation to obtain an estimate for the parameters 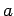 and
and 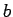 .
.



Next: Exercise 4.6: Ground state
Up: Evaluation of observables
Previous: Evaluation of observables
Adrian E. Feiguin
2004-06-01