


Next: MD simulation of Hard
Up: Tricks of the trade
Previous: Starting configuration
Since the number of particles is usually fixes and conserved, the way to
achieve the desired density os to adjust the volume. This is done scaling
all coordinates by a suitable factor.
In Molecular Dynamics, the temperature is a measurable quantity. Kinetic
theory tells us that
where 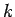 is the Boltzmann's constant, and
is the Boltzmann's constant, and
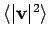 is the averaged square of the velocity. In a simulation, we have to average
this quantity over a number of MD steps to get a measurement of
is the averaged square of the velocity. In a simulation, we have to average
this quantity over a number of MD steps to get a measurement of 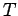 . If we
want to simulate a particular temperature, we rescale each component of the
velocity vector of every particle by
. If we
want to simulate a particular temperature, we rescale each component of the
velocity vector of every particle by
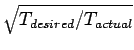 .
Obviously
.
Obviously 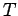 is a fluctuating quantity and can therefore be adjusted only
approximately.
is a fluctuating quantity and can therefore be adjusted only
approximately.
Adrian E. Feiguin
2004-06-01
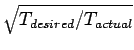 .
Obviously
.
Obviously