 |
 |
Since the size of our system is typically 10-100 molecular diameters, this
is certainly not a good representation for a macroscopic sample because most
of the particles will be situated near a ``wall'' or ``boundary''. To
minimize the effects of the boundaries and to simulate more closely the
Since the size of our system is typically 10-100 molecular diameters, this
is certainly not a good representation for a macroscopic sample because most
of the particles will be situated near a ``wall'' or ``boundary''. To
minimize the effects of the boundaries and to simulate more closely the
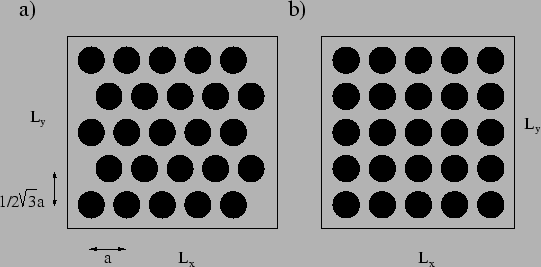
Picking the right starting configuration is not trivial. The first choice
would be to place the molecules randomly distributed, but this would give
rice to large starting energies and forces, since many pairs would be placed
at unphysical short distances. It is therefore customary to place the
particles in the vertices of a some crystal lattice (face centered cubic
-or triangular-, for instance). The ![]() and
and ![]() components of the
velocities can be picked randomly in an interval
components of the
velocities can be picked randomly in an interval
![]() .
Before starting the proper simulation and the measurement of the physical
quantities, it is necessary to perform a ``thermalization'' run to let the
system relax to a situation of dynamical and thermal equilibrium.
.
Before starting the proper simulation and the measurement of the physical
quantities, it is necessary to perform a ``thermalization'' run to let the
system relax to a situation of dynamical and thermal equilibrium.