Human Subject Protection Training
Required human subject research training
Under the direction of the Office of the Vice Provost for Research, Northeastern University requires completion of training on the protection of human subjects and the ethical principles of research for all human subject research (HSR), regardless of whether or not investigators have received funding to support their project. This training is mandatory for all faculty, staff, and students who conduct/supervise HSR whether on campus or off-campus.
If you have completed HSR training, please provide documentation. Documentation is only required to be submitted once and may be provided with your IRB protocol application. The Office of Human Subject Research Protection (HSRP) will accept documentation of human subject protection training from other institutions. If you have not yet completed some type of human subject protection training, Northeastern University has an account with CITI. All NU affiliates can take this course at no charge. Please note that approval for training is for 3 years. Any training that was completed more than 3 years ago will not satisfy NU IRB training requirements. Those who continue to be engaged in HSR will be instructed to complete a refresher course.
The HSRP requires completion of the training module titled CITI Human Subjects Research Stage 1 Basic Course. Note that the Responsible Conduct of Research trainings do not satisfy IRB training requirements.
INFORMATION ABOUT HOW TO ACCESS AND COMPLETE THE CITI TRAINING
For new users:
| For users with an existing CITI account:
|
When you submit a study for review, you will need to include your Human Subjects Research course completion date.
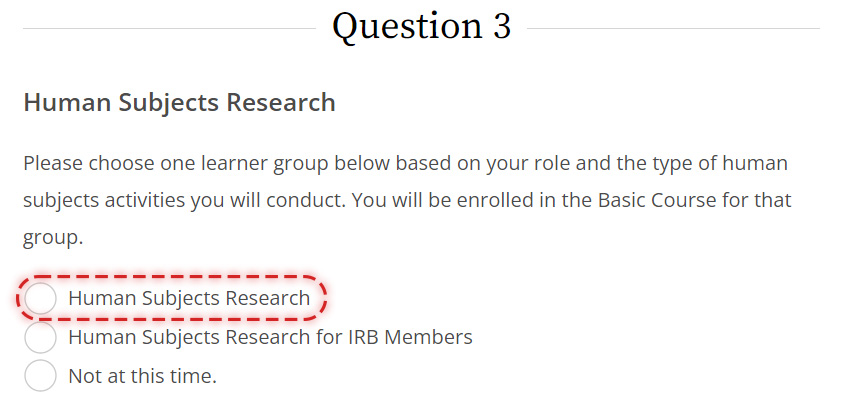
Please be sure to select the correct course circled below when registering for IRB CITI training under Question 3: