|
Lithium Ion Battery Materials
LEAP is currently at the forefront of two important initiatives which have the potential of providing significant boost to the current state of the art.
These are:
(A) Development of Li-Air battery materials, which includes electrolyte membrane separator and air cathode materials to improve capacity and rechargeability of these systems
(B) Development of next generation of cathode intercalation materials with high rate capability
and safety designed for automotive and other high rate applications:
Li-Air Battery Materials Effort:
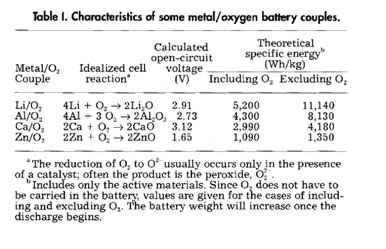
As shown in the Table below, the theoretical specific energy density of Li-oxygen cell is 13.0 KWh/Kg, the highest for a metal air battery. It is also environmentally friendly, possesses a flat discharge profile and is corrosion resistant enabling long shelf life. The advantage of Li is readily clear from comparison with other possible metals due to its relative low weight. These batteries originally reported by Abraham in 1996 (1) had ascribed Li2O2 (2Li + O2 Li2O2; E°= 3.1 V) as the discharge product consistent with discharge cell voltage of 2.9V. However two other products are possible based on their theoretical potentials (1-3). These are Li2O (4 Li + O2 ® 2 Li2O; E°=2.91 V) and LiO2 (Li + O2 ® LiO2; E°= 3.0 V). As reported recently by us (2), the choice of cations affects the stability (half life) of the discharge product. The first product of the reduction of oxygen is the one electron reduction product superoxide (Li + O2 + e- → LiO2), which as reported recently by us (2) in the case of bulky cations (Bu4N+) is extremely stable and resists further reduction to peroxide and oxide ( and ). In the presence of Li ions, the superoxide has a relatively short lifetime (5 to 10 minutes) and reduced further to peroxides and oxygen according to equation 2 LiO2 → Li2O2 + O2. Recent data also suggests that the formation of Li2O is possible (Li2O2 + 2 Li+ + 2 e- → 2Li2O) from the reduction of the peroxide and this is intimately associated with the choice of electrolyte solvent and the catalyst present. Overall goal of this effort is to provide fundamental understanding of the challenges for successful development of rechargeable Li-Air batteries.
References
(1) K. M. Abraham and Z. Jiang, Journal of The Electrochemical Society, 143, 1 (1996).
(2) C. O' Laoire, S. Mukerjee and K .M. Abraham, E. Plichta and M.A. Henderson, J. Phys. Chem C, 2009 (in press, available online).
(3) A. Debart, A.J. Paterson, J.L. Bao, P.G. Bruce, Angew. Chem. Int. Ed. 47, 4521 (2008).
Development of Advanced Cathodes for Li-ion Batteries
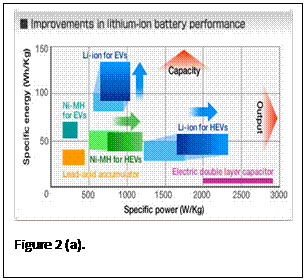
A paradigm shift in the current state of the art battery technology is required to enable progress in electric and plug-in-hybrid electric vehicles (EV and PHEV). It is recognized that only a Lithium-ion battery (LIB) is capable of providing the all electric driving range of EV and PHEV (see Fig. below). However, today’s LIBs fall short in energy density, cost and safety to make electric vehicles attractive to average US consumers. The LIB cells available today have specific energies under 200 Wh/kg. A fully packaged 50 kWh EV battery built from these cells for 250-300 mile all electric drive would weigh nearly 1000 pounds. Battery weight is also an issue for the 15-20 kWh PHEV batteries built with today’s LIB cells. The need to achieve battery specific energies greater than 300 Wh/kg and specific power exceeding 2kW/kg are paramount in realizing wide-spread electrification of automobiles (Fig. 2). Major improvements in LIB specific energies can be addressed with novel concepts for LIB chemistries. The cost and safety of the batteries must also be addressed in parallel with increases in energy density. The development of alternative vanadium phosphate based cathode electrode materials in conjunction with advanced separator materials will greatly enhance both performance and safety of LIBs. Substituting the proposed vanadium electrode materials will reduce cost as well since the cathode material represents about 40% of the cost of LIB cells.
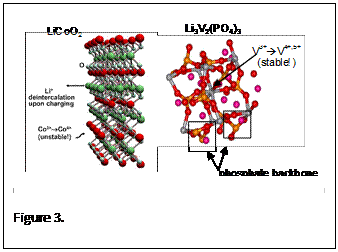 These lithium-ion battery systems will overcome the main drawbacks of fast recharge, safety hazards, cost and energy density that exist in the current battery technology. This disruptive technology will unseat the current standard lithium cobalt oxide cathode material due to its safety features (Fig 2), while still improving the power and energy storage, unlike the current state of the art lithium iron phosphate, which only addressed the safety and rate capability. Another advantage of these materials over the lithium cobalt oxide based batteries is that they can be recharged and charged at very high rates. The combined safety and high-rate capability features make for an excellent battery candidate for PHEV and EV applications. The disruptive nature of this technology will allow for a dramatic increase of the all-electric mileage for PHEV vehicles, a great improvement in the reduction of emissions/carbon footprint, and will play a large role in reducing USA’s dependence on importing foreign oil. The key disruptive features here are the significant leap in energy density above even the best iron-magnesium phosphate cathode compound as seen in figure 3, where there is greater than a 100 Wh/kg improvement in performance over 500+ cycles. The improved discharge potential profile for Lithium vanadium phosphate has shown a 40% increase in stored energy over lithium iron phosphate. In figure 3, the large increase in operative voltage for lithium vanadium fluorophosphate can be observed as compared to the current state of the art lithium iron phosphate as well as the current standard lithium These lithium-ion battery systems will overcome the main drawbacks of fast recharge, safety hazards, cost and energy density that exist in the current battery technology. This disruptive technology will unseat the current standard lithium cobalt oxide cathode material due to its safety features (Fig 2), while still improving the power and energy storage, unlike the current state of the art lithium iron phosphate, which only addressed the safety and rate capability. Another advantage of these materials over the lithium cobalt oxide based batteries is that they can be recharged and charged at very high rates. The combined safety and high-rate capability features make for an excellent battery candidate for PHEV and EV applications. The disruptive nature of this technology will allow for a dramatic increase of the all-electric mileage for PHEV vehicles, a great improvement in the reduction of emissions/carbon footprint, and will play a large role in reducing USA’s dependence on importing foreign oil. The key disruptive features here are the significant leap in energy density above even the best iron-magnesium phosphate cathode compound as seen in figure 3, where there is greater than a 100 Wh/kg improvement in performance over 500+ cycles. The improved discharge potential profile for Lithium vanadium phosphate has shown a 40% increase in stored energy over lithium iron phosphate. In figure 3, the large increase in operative voltage for lithium vanadium fluorophosphate can be observed as compared to the current state of the art lithium iron phosphate as well as the current standard lithium
|