About Northeastern
A global research university, powered by experience
Experience is at the heart of everything
we do
Founded in 1898, we’re renowned for our experiential learning model, high-impact research, deep partnerships, and worldwide reach. From day one, we’ve pursued innovative ways of teaching and research that place a premium on experience and engagement with the world. Today, our signature approach erases traditional boundaries, empowering not only students, but faculty, alumni, partners, and innovators to solve problems and pursue impact.
Opportunity is always in motion
We’ve created a one-of-a-kind global university system of 13 campuses, 49 alumni communities, and 3,500+ employer partners that forms a dynamic, diverse, and inclusive community. We create new opportunities for richer educational experiences and deeper collaborations across industries and disciplines to bring the right expertise together to go from promise to reality.
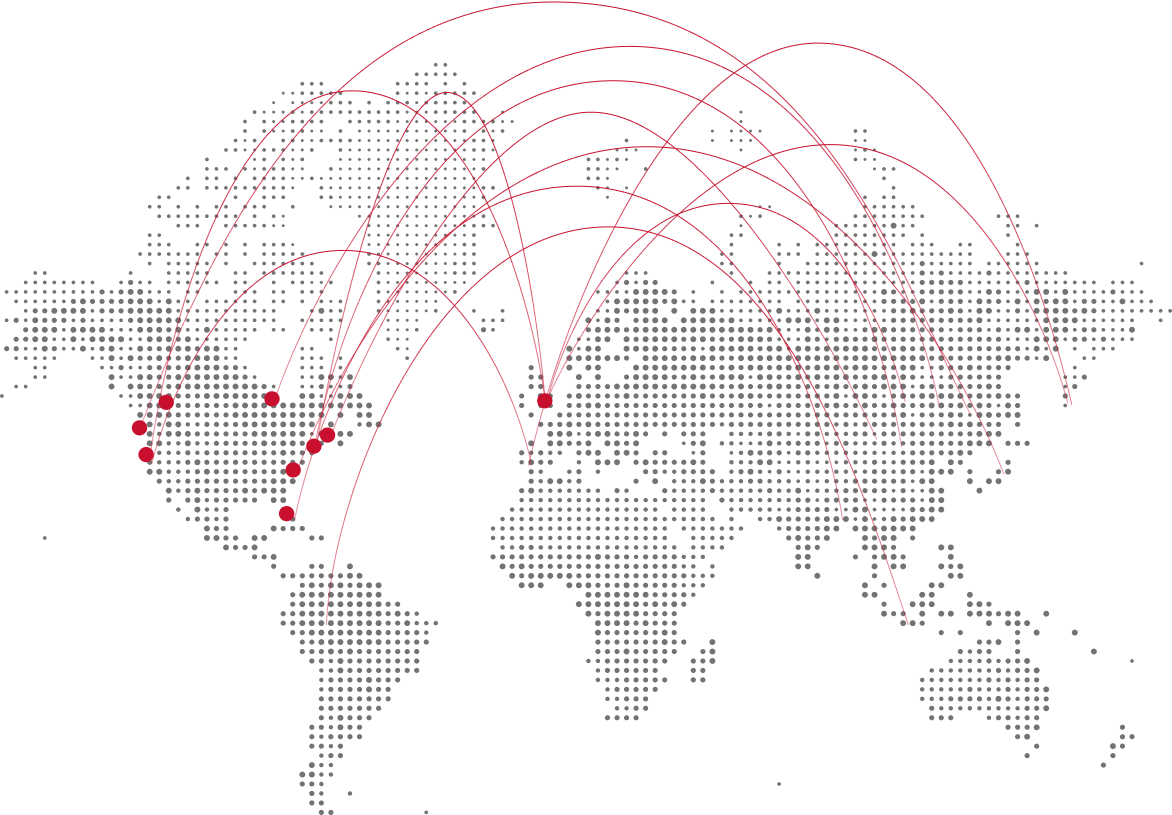
Our campuses
Find unique opportunities for experience-powered learning and discovery.

The leader in global experiential learning
Providing you with the world’s most dynamic, rich, and global undergraduate, graduate, and doctoral education
Research for global impact
Building powerful interdisciplinary teams of faculty and partners to solve widespread, global problems

Meet our president
Joseph E. Aoun, an internationally renowned scholar in linguistics, is a leading authority on global and experiential learning and is at the forefront of driving innovation in higher education. He is the author of Robot-Proof: Higher Education in the Age of Artificial Intelligence, a blueprint for the future of learning.
Big ideas, big results
93%
of graduates employed full time or enrolled in graduate school within nine months of graduation (multi-year average since 2006)
$450M
in financial aid grants for 2023–2024
$282.4M
in external research awards for 2022–2023, up 480% since 2006
2,400+
employer partners hire our undergraduate and graduate students for short-term projects that contribute to their organization

















