Meters and kilograms are not meant to measure molecules. Using these units
would imply the clumsy manipulation of small numbers. We want to choose
units of mass, energy, and length such that the values of the quantities are
always of the order ![]() . For instance, in the case of the Lennard-Jones
potential is wise to use
. For instance, in the case of the Lennard-Jones
potential is wise to use ![]() as a unit of length,
as a unit of length, ![]() as a
unit of energy, and
as a
unit of energy, and ![]() as the unit of mass. Re-scaling by these
quantities, the potentia would be
as the unit of mass. Re-scaling by these
quantities, the potentia would be
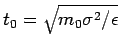 . For the electrical charge, the natural unit is the charge
of the electron
. For the electrical charge, the natural unit is the charge
of the electron