


Next: Tricks of the trade
Up: Molecular Dynamics
Previous: Molecular Dynamics
Our first goal is to identify the model we want to simulate. The first
simplification is to consider the molecules spherical, chemically inert, and
that they move according to the laws of classical mechanics. We also assume
that the force between molecules depends only on the distance between them.
The form of this potential for electrically neutral atoms can be constructed
from a detailed first principles quantum mechanical calculation. Such
calculation can be complicated, so we will consider a phenomenological form.
The most important features are: strong repulsion at short
distances, weak attraction at large separations. The repulsion originates
from the electrostatic interaction between equally charged particles, and
the long range attraction from the mutual polarization of the electronic
clouds, known as ``van de Waals'' force.
Below we list some model potentials:
| Hard Spheres |
|
| First approximation |
|
| in many applications |
|
|
| Lennard-Jones |
![$V(r)=4\epsilon \left[ \left( \frac{r}{\sigma }\right)
^{-12}-\left( \frac{r}{\sigma }\right) ^{-6}\right] $](img320.png) |
| Noble gas atoms; |
|
| nearly spherical |
|
| molecules |
|
|
| Harmonic |
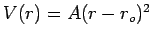 |
Intramolecular bonds |



Next: Tricks of the trade
Up: Molecular Dynamics
Previous: Molecular Dynamics
Adrian E. Feiguin
2004-06-01
![$V(r)=4\epsilon \left[ \left( \frac{r}{\sigma }\right)
^{-12}-\left( \frac{r}{\sigma }\right) ^{-6}\right] $](img320.png)
![$V(r)=4\epsilon \left[ \left( \frac{r}{\sigma }\right)
^{-12}-\left( \frac{r}{\sigma }\right) ^{-6}\right] $](img320.png)