


Next: Temperature and the Canonical
Up: One-dimensional Classical Ideal Gas
Previous: One-dimensional Classical Ideal Gas
- Write a program to simulate a 1D ideal gas using the demon
algorithm.
- Use the initial condition that all particles have the same velocity
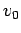 .
The mass of the particles is set to unity. Choose
.
The mass of the particles is set to unity. Choose 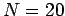 , total energy
, total energy
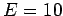 , a maximum velocity change
, a maximum velocity change 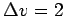 , and 50 steps for the
simulation.
Increase the number of MC steps per particle until the desired averages
are constant within a desired range.
, and 50 steps for the
simulation.
Increase the number of MC steps per particle until the desired averages
are constant within a desired range.
- What is the initial mean velocity per particle? What is the
equilibrium mean velocity per particle?
- Compute the mean energy of the demon and the mean system energy per
particle. Do your results in parts 3 and 4 depend on whether the particles
are chosen randomly or sequentially?
- Choose
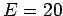 and find the value of the maximum velocity change
and find the value of the maximum velocity change
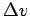 that
yields an acceptance rate of
that
yields an acceptance rate of 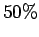 . Compute the mean demon energy and
mean system energy per particle after equilibrium has been established.
Then consider
. Compute the mean demon energy and
mean system energy per particle after equilibrium has been established.
Then consider 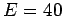 and obtain an approximate relation between the mean
demon energy and the mean system energy per particle.
and obtain an approximate relation between the mean
demon energy and the mean system energy per particle.
- The MC simulation in the microcanonical ensemble is done at a fixed
total energy with no reference to temperature. Determine the temperature by
the relation
Set the Boltzamann's constant to unity. Use this relation to obtain 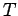 .
Is
.
Is 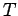 related to the mean demon energy?
related to the mean demon energy?
- What is the meaning of the ``acceptance ratio''? What is the
empirical relation between the acceptance ratio and
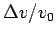 ?
?



Next: Temperature and the Canonical
Up: One-dimensional Classical Ideal Gas
Previous: One-dimensional Classical Ideal Gas
Adrian E. Feiguin
2004-06-01